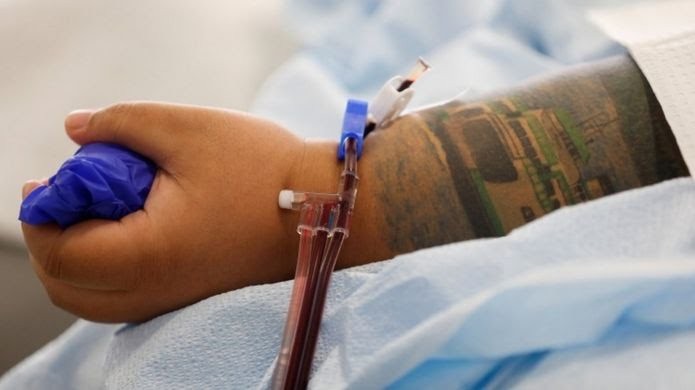
The technique uses antibody-rich blood plasma from people who’ve recovered from the disease, and has already been used on over 70,000 people in the US.
The FDA says early trials indicate it is safe, although more are needed to prove effectiveness.
Several experts have questioned the robustness of studies into its use.
The announcement came a day after President Donald Trump accused the FDA of impeding the rollout of vaccines and therapeutics for political reasons, and on the eve of the Republican National Convention, where Mr Trump will launch his campaign to win a second term in the White House.
This is what I’ve been looking forward to doing for a long time,” the president told reporters on Sunday.
“I’m pleased to make a truly historic announcement in our battle against the China virus that will save countless lives.”
Mr Trump described the procedure as a powerful therapy, as he appealed for Americans to come forward to donate plasma if they’d recovered from Covid-19.
More than 176,000 people have died from coronavirus since the start of the outbreak in the United States, according to a tally by Johns Hopkins University. Nearly 5.7 million cases have also been confirmed nationwide. The country has had more confirmed cases and deaths than anywhere else in the world










